I’ve concluded that Hibiscus dasycalyx, the Neches river rose mallow, is certainly in danger of being lost in the wild. Intrusion by other mallows seems to favor the invader, whether H. moscheuotis, H. militaris, or H. laevis. Where they comingle come progeny sharing a fairly friendly gene pool. It’s a modest megacharasmatic endangered plant with a neat story. Known in the wild from only four populations in East Texas, it must be noted that the species has found a home in over a dozen reintroduction projects, as well as in Southern Arboretums, botanical gardens and home landscapes. While no barn burner in the market, it has moved into all kinds of horticultural and nursery channels across the South. Along the way, as professional and amateur horticulturists and breeders are known to do, it’s been used to produce a range of interesting hybrids. The long narrow leaves are unique and incorporating that aesthetic into progeny with large red, pink, or white flowers, well, it’s done. Given half a chance, this wetland species will survive in a home landscape. To be totally cheerful, all it needs is a naturally wet site, good sun, and you can’t let the spot grow into a forest.
First a little botany: Hibiscus dasycalyx is a shrubby-appearing herbaceous perennial that grows 0.8-2.3 meters in height. The glabrous leaves are narrow and deeply lobed, and the flowers are white to cream with a crimson throat. The calyx is densely covered with long hairs, and the seeds are densely reddish hairy. The specific epithet “dasycalyx” translates from the Greek to mean a fruit-covering that is “shaggy” or “thick-haired”. Blake (1958) commented that the species is distinguishable from all other United States Hibiscus spp. because of its densely spreading hirsute calyx. An obligate wetland species (Reed 1986), H. dasycalyx is a member of Section Muenchhusia (n=19), a group of five closely related Hibiscus species that are uniquely designated “rose mallows” (Blanchard 1976). The rose-mallows may have evolved recently and all are in floodplain or coastal plain wetland habitats of the eastern U. S., the western boundary being East-Texas north to Canada. Seed dispersal for this group appears to be entirely water-dependent.

Hibiscus dasycalyx in flower July 2017

Diversity in the plot: H. lavevis (?) and H. dasycalyx.
Rarity: Ecological and Genetic Status
The four known locations of H. dasycalyx are wetland habitats within floodplain plant communities of large creeks (Houston Co. and Cherokee Co.) and in major river bottoms (Trinity Co.). These plant community types have been termed by Nixon (1985)
as Wet Creek Bottom and River Bottom Communities. Within these communities, Hibiscus dasycalyx occupies open slough and oxbow habitats that experience periodic flooding. The Trinity County site is on the Neches River floodplain and was discovered by Ivan Shiller in 1955. This original site was later designated the type locality by Blake (1958). In 1978, 23 years later, a second population was found by Claude McLeod on Tantaboque Creek, a tributary of the Trinity River in Houston County.

Old image of Jeff Williams, SFASU ATCOFA, and a colony near Ponte, Texas
In 1988, when only two locations were known, investigators found that the type locality of H. dasycalyx had already been severely disturbed (McMahan 1988). Paul Cox (1988) and Elray Nixon reported that “all but two plants had been dredged up from an expanded drainage project and were resprouting in a soil bank about three meters above the former water level”. The third documented population on Mud Creek, a tributary of the Angelina River in Cherokee County, was found in 1992 by Jason Singhurst (Carr 1992). It too has suffered over the years (mowing, herbicides, etc.).
In the early 1990s it was noted that all four populations are in serious decline. Surveys performed by TNHP staff and Warnock in slough and oxbow habitats have produced negative findings (Orzell 1990, Warnock 1995). Over the past years, cursory searches have been conducted in potential habitat around highway-river crossings in at least 35 counties. Habitat reduction by drainage and filling of sloughs and oxbows, prevention of oxbow production, and channelization have likely negatively impacted H. dasycalyx populations (Warnock 1995). In addition to habitat reduction, genetic drift appears to be a major threat to this extremely rare species. The two wide-spread and closely related H. laevis and H. moscheutos are found growing in the same or nearby wetland habitats. Apparent hybrids with H. moscheutos have been found at the Trinity Co. and Houston Co. populations. Hybrids with H. laevis have also been noted at the Cherokee Co. site.
Orland Blanchard (1976) conducted thesis research on the Hibiscus section Trionum Sensu Lato in 1976. Blanchard’s research included morphological descriptions, systematics, hybridization studies, and distribution/habitat information for H. dasycalyx. In 1992, Klips hypothesized that H. dasycalyx is a product of diploid hybridization speciation between two wide-spread sympatric closely related species, H. laevis All. (halberd-leaf rose-mallow) [syn. H. militaris Cav.] and H. moscheutos. Klips (1995) studied morphological relationships and conducted electrophoretic screening on the three species and determined that H. dasycalyx is probably not the product of hybridization but perhaps a recent offshoot from the interfertile and morphologically similar H. laevis. The genetic relationships among these three Hibiscus species and the potential for current and future genetic-swamping of the rare H. dasycalyx has been reported (Blanchard 1976, Klips 1995, Warnock 1995). Despite these questions, many investigators continue to recognize H. dasycalyx as a distinct species as concluded in early work (Gould 1975, Correll & Johnson 1979, Nixon 1985). The debate remains.
RELATED RESEARCH
Research on H. dasycalyx has been conducted by Texas-based agencies and institutions, including the Texas Natural Heritage Program (TNHP), the Texas Nature Conservancy (TNC), the CPC at Mercer Arboretum and at the San Antonio Botanical Gardens (SABG), the Texas Regional Institute for Environmental Studies (TRIES, Sam Houston State University), the Stephen F. Austin State University (SFASU) Arboretum and the USFWS in Houston and Austin. These organizations have reported on the status of survey, habitat and plant vigor, and/or propagation of H. dasycalyx (Cox 1988, Orzell 1990, San Antonio Botanical Center 1990, Smith & Creech 1995). Blanchard (1976) and the San Antonio Botanical Center (1990) have reported successful cultivation of H. dasycalyx. Likewise, Erin Smith, SFASU graduate research assistant, conducted rooting experiments from stem cuttings, seed germination and fertilization trials in 1994 and 1995 (Smith & Creech 1995). Additional stem-cutting propagation and fertilizer trials were conducted by the author in 1995 on cultivated plants of H. dasycalyx. In general, these studies indicated favorable rooting percentages of softwood cuttings under mist with photoperiod interruption.
A variety of ecology research has been published on other rose-mallows. This information can be useful in the formulation of a conservation strategy for H. dasycalyx because of the similarities in habitat niche and reproductive strategy of these species. Genetic affinities between H. laevis and Florida-endemic H. coccineus have been studied by Wise and Menzel (1971), elucidating a lineage with H. dasycalyx. Also important is the fact that all of the rose-mallows appear to be pollinated primarily by Ptilothrix bombiformis Cresson (Anthophoridae), a non-social bee that is a Hibiscus specialist (Blanchard 1976; Spira 1989). Therefore, study of the pollination ecology of other rose-mallows may be pertinent to the understanding of H. dasycalyx’s current status and future chances as a self-sustaining species. Research on the pollination ecology (Spira et al. 1996, Spira et al. 1992), pollen competitive ability (Snow & Spira 1994), selfed progeny vigor (Snow & Spira 1993), seed predation and reproductive success (Spira 1987) has been conducted for the wide-spread rose-mallow, H. moscheutos. In addition, dissertation research is available on the community production and biomass allocation of H. moscheutos (Cahoon 1982) as well as on the distribution, herbivory, and seed set problems of southeastern Hibiscus spp (Cochis 1964; Robbins 1985). Observations from these investigations can guide the development of appropriate strategies for the augmentation of H. dasycalyx at in-situ and ex-situ locations.
Cultivation research has been conducted on other rose-mallows as well. H. moscheutos has been cultivated for ornamental use in the United States since the mid 1800’s (Welch & Grant 1995). For the establishment and maintenance of cultivated H. moscheutos, which is a heavy feeder, an annual application of a balanced fertilizer is recommended (Giles et al. 1980). Propagation and horticultural care information on both H. moscheutos and H. laevis (syn. H. militaris) can be found ubiquitously in gardening literature (Griffiths et al. 1992). The Landscape Restoration Handbook lists these two rose-mallows as native species suitable for introduction into marshy habitats in the Southeast U. S. (Harker et al. 1993). The Handbook provides information on plant type, environmental tolerances, aesthetic value, wildlife value, flower color, bloom period, and landscape use for over 3300 native plant species.
Conservation and Recovery Strategies
Prior to the addition of H. dasycalyx to the Category 1 candidate list, Warnock (1995) indicated that there were no mechanisms for protection of this rose-mallow since known populations are subject to mowing, discing, bulldozing, herbicide use, drainage, genetic swamping from sympatric rose-mallows, genetic drift and, potentially, are subject to over-collecting. Unless the species is listed as endangered or threatened, either at the state or federal level, the remaining H. dasycalyx plants and its habitat cannot be protected or managed for conservation (Warnock 1995).
Listing a rare plant species is not a panacea, however. The 1973 Endangered Species Act (ESA) may prevent the federal government and private citizens from harassing or killing listed animal species on both public and private land, but there are no prohibitions from destroying threatened and endangered plant species by private citizens on private land (Doughty & Parmenter 1989). Since the federal government is prohibited from the extirpation of threatened and endangered plants, federally funded or managed projects are required to mitigate activities to ensure conservation. Because the majority of the known occurrences of H. dasycalyx are found on private land, listing by the federal government may have little effect on the destiny of this species. Listing or not, the key to recovery of this Hibiscus is the implementation of cooperative agreements among federal and state agencies, institutions, and private land owners.
While it was once the priority of the USFWS to explore conservation possibilities that could avoid the listing of H. dasycalyx as endangered (Nemec pers. comm. 1996), the species is now listed. While rare plant management can involve many different methods, there is an increasing need for population re-introduction and restoration to be a part of the recovery plan (Falk 1987). Re-introduction is defined as the intentional establishment of a plant species where it has become extirpated for the purpose of establishing a self-sustaining population (Maunder 1992). In order to implement re-introduction and restoration, cultivation studies need to be implemented. It is obvious that the primary goal of conservation research on a given species is not horticultural knowledge, but such knowledge is necessary to develop techniques for cultivating rare species as a means to an end (Affolter 1997).
For H. dasycalyx, conservation strategies recommended by Warnock (1995) include the cultivation of plants to ensure against catastrophic loss, to prepare for re-introductory work, and to provide for scientific and educational activities. However, Ashton (1987) asserts that genetic viability of remaining populations of rare plants must be estimated before a conservation strategy is executed. This research requires funding. It is unknown when or if H. dasycalyx will be federally listed and when funding will be allocated for genetic viability research and conservation strategy design, initiation and implementation. While genetic viability is being ascertained for any rare species, immediate propagation and development of controlled ex-situ sites can check over-collecting due to potential horticultural value and curb destructive and unethical collection into the future (Mc Cartney 1995). As for H. dasycalyx, it appears that ex-situ preservation measures need to be taken soon since habitat preservation is tenuous for the three existing populations. Species such as H. dasycalyx, which have fewer than five locations, may become extinct if a chance event causes a population crash (Falk 1992). In addition to the threat of continued habitat degradation, Klips (1995) suggests that H. dasycalyx should be isolated from H. laevis and H. moscheutos to avoid dilution by gene flow when establishing preserves. Since hybridization is occurring at all three of the natural populations, this recommendation excludes them as re-introduction sites. In the interim, Mill Creek Gardens can provide a safe ex-situ site for cultivated H. dasycalyx because the site is isolated from all other Hibiscus species. Continued propagation and monitoring would be under the auspices of the Stephen F. Austin State University Arboretum. Since funding for the implementation of conservation projects is affected by federal, state, and industry politics, many plans are not implemented or can occur piecemeal over time. Augmentation via propagation and establishment in ex-situ sites needs to be initiated while waiting for the necessary bureaucratic processes to occur.
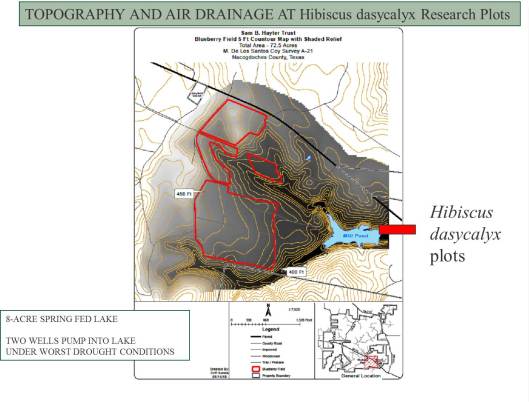
RESEARCH PLOTS, NACOGDOCHES, TX
Experimental Design
A randomized complete block design (RCBD) was used for this H. dasycalyx fertilizer and mulching trial. Plants were randomly divided into groups of four and randomly assigned to three blocks of eight groups each. 32 plants were planted in each of three blocks, giving a total of 96 plants. Treatments were randomly assigned to 24 experimental units (plots) containing four plants each, with each treatment appearing exactly once in every block. Between the four rates of fertilizer and two mulching regimes, there are eight different treatments per block. The blocks are 6.1 by 12.2 meters (20 by 40 feet). The plots are 3.05 by 3.05 meters (10 by 10 feet).
Broad overview of the terrain adjacent to plots
In September of 1995, 96 plants were selected from several hundred plants using guidelines regarding morphologically correct features for the species. A portion of the H. dasycalyx plants used were stem cuttings taken from a single plant that had been propagated from the type locality and grown at the SFASU Arboretum. The rest of the plants used were progeny of these cutting-grown plants. The seeds were germinated and the cuttings were rooted and grown in the SFASU Arboretum greenhouse; then hardened off in one gallon cans in an outdoor shade house from 1994 to 1995. A selection protocol was followed because putative hybrids with H. moscheutos occur at the type locality. Appendix 3 provides a comparison of morphological features among H. dasycalyx, H. moscheutos, and H. laevis (Warnock 1995).
The fertilization and mulching experiment on H. dasycalyx at the Mill Creek Gardens research site was initiated in October of 1995 and concluded in November of 1996. Two separate applications at four rates of slow release 14-14-14 OsmocoteÒ fertilizer was made during the trial, once during planting (October 18, 1995) and again, approximately one month after dormancy-break (March 21, 1996). The slow release fertilizer is encapsulated within multiple layers of polymeric resin. When water vapor penetrates the permeable shell and dissolves the nutrient core, the resulting osmotic pressure within the granule meters the liquid nutrients through the coating and into the surrounding soil/media (Scotts 1995). 14-14-14 OsmocoteÒ is coated to provide approximately 4 months, based on an average soil temperature of 70°F. Some use can occur in the winter months by an herbaceous perennial such as H. dasycalyx, via absorption and storage in the roots.

Research plots
Treatments
The fall application (10/18/95) of the four rates (0, 18, 36, and 72 g) was applied in-ground and as a top-dress (total application was 0, 36, 72, and 144g). The fertilizer was mixed into the soil/mulch directly below the roots as the plantings were made. The top-dress was applied within the root zone (10 cm radius) of the plants. The spring application (3/21/96) was a repeat of the 0, 18, 36, and 72 g rates as a top-dress only. Therefore, total fertilizer application for the study was 0, 54, 108, and 216g per plant. In the dry soil plots “wells” of soil/mulch were constructed around the root zones. In the standing-water plots plastic collars were placed around the plants to hold the fertilizer prills within the root zones. To further avoid intra-specific interaction of root zones and fertilization, the plantings were made at least a meter apart.
The low application rate (54g/plant) is equivalent to applying 12lbs of fertilizer per 100 plants or 1.9 ounces per plant. In terms of pounds per square-foot, the low rate is equivalent to .36 lbs/ft2. For 14% NPK, the low application rate is approximately .05 lbs/ft2 of each of the nutrients.
For plots selected to receive mulch, two gallons were mixed in the planting hole and four gallons of mulch was applied as top-dress around the plant. All holes were dug uniformly to accommodate the addition of mulch. For each non-amended plant, the soil was simply returned to the planting hole with the appropriate rate of fertilizer.
Plants were watered at the end of planting time (10/18/95) and then again on 10/28/95 due to drought conditions. Weed removal was conducted once during the study. In mid September 1996, all plants were removed within a 20 cm radius of each H. dasycalyx, regardless of their perceived threat.
Results
Stacy Scott collected data for three years (1996, 1997 and 1998). Results concluded that mulch and fertilizer (applied at planting) improved plant performance three years in a row.

Influence of fertilizer, mulch, and soil condition on growth, stem number and capsule number

Influence of fertilizer on mulched and non-mulched plants
We concluded fertilizer treatments did help plant growth and long term survival. Mulch did not. What was most interesting is that even though fertilizer was applied only in the first year, the benefits of that application were still significantly apparent three years later. Perhaps the additional growth in the first year allowed the fertilized plants to compete with weeds later in the plant’s life.
RESEARCH PLOT REVISITED IN 2013
Dr. Dave Kulhavy and students revisited the plots in 2013 and mapped the plants. Data collected included plant height and number of stalks per plant.

Number of Stalks and Plant Height distribution
With this data, Dr. Kulhavy and students placed plants in categories based on stalk number. Because stalk number is some indication of plant size and age, it could be demonstrated that in nearly twenty years, the plants have a fluid presentation across the site.

Dr. Dave Kulhavy and students in summer of 2013 mapping the colony

Distribution of plants by number of stalks in 2013

Large plants inside and outside the original plots.
GENETIC VIABILITY RESEARCH NEEDED
A major issue surrounding the propagation and re-introduction of extremely rare species such as H. dasycalyx is the small number of plants that exist. Would re-introduction involving the propagation of a few individuals (a narrow genetic base) warrant the time and expense of such a project? The CPC (1991) recommends that between 10 and 50 individuals could preserve a significant fraction of the genetic information within that population. As of 1995, the three populations of H. dasycalyx contain as few as several to perhaps no more than 60 plants, and because each of the populations are hybridizing with H. moscheutos or H. laevis, it is likely that this sampling criteria will not be met for this species. Some investigators suggest that dozens of genetic individuals are needed to preserve fit genotypes in progeny (Guerrant 1992; Ashton 1978). However, this assertion may be based on theoretical assumptions or on animal studies and does not necessarily characterized long-term or species-specific plant studies (Eloff and Powrie 1990). New populations of plants are often started by one or several founding individual(s) and have become genetically viable and self-sustaining. One example cited by Eloff and Powrie (1990) involved the establishment of three plants of Aloe spectabilis (Aloeaceae) transplanted in 1900 to a location genetically isolated from any colony of Aloe. The original three plants produced a population whose numbers have increased to about 10,000 plants. These investigators indicate that at least with Aloe spectabilis, three plants were sufficient to establish a self-sustaining population with apparently the same diversity as the original colony.

Amber Miller, USFW, during a July 6, 2017 survey of the reintroduction plot
Even if the CPC’s recommended sampling criteria is met, it is argued that ex-situ conservation of a small genetic sample can lead to unpredictable changes in gene frequencies and loss of gene combinations (Ashton 1978). Some investigators assert, further, that the use of genetically “depauperate” or cloned material will doom a project because of the genetic homogeneity of the re-introduced individuals (Maunder 1992; Gordon 1994). There is no question that artificial augmentation terminates the natural selection process, but is it necessarily true that the use of small samples causes deleterious or undesirable gene combinations? Stebbins (1950) states that, as a matter of course, naturally rare plant species evolve thoroughly exposed to inbreeding and that depression is unlikely to occur. The answer is species and strategy specific. More research is needed in the arena of founder effects and population bottlenecks involving naturally rare plant species before decisions are made to forego conservation of them. An excellent examination of extinction probability and minimal viable population (MVP) regarding the conservation of rare plant species can be found in Conservation Biology (Fiedler and Jain 1992).
CONSERVATION HORTICULTURE
Closer to the focus of this research is “conservation horticulture,” defined by Affolter (1997) as the application of techniques and the knowledge base of horticulture to rare plant conservation. The technical aspects of a re-introduction project for endangered plant species must be logistically feasible or the effort is academic (Falk and Olwell 1992). The technical feasibility questions posed by Falk and Olwell (1992) are: Is there enough re-introduction material? Are propagation and transplanting techniques being studied? What are the interim management needs of newly transplanted material? Horticulturists have begun to respond to the concerns of managers who are struggling with the huge task of rare plant management. The International Conference on Botanic Gardens and the World Conservation Strategy (Bramwell 1987) encouraged the involvement of botanic gardens in conservation action; botanic gardens should maintain, propagate and make available stock of threatened species for scientific and horticultural research. The International Conference stated that no single approach to the conservation of endangered species can be relied upon; therefore, ex-situ conservation is a necessary adjunct to in-situ conservation (Bramwell 1987). Given (1987) lists five reasons why botanic gardens and arboreta should grow plants for conservation purposes (p.104):
To have as many threatened species in cultivation as practicable as an insurance against their loss in the wild.
To cultivate critically threatened species in sufficient numbers so as to prevent significant genetic erosion.
To have material available for research and for assessment for economic use.
To have collections of plants available for educational programs and for public displays.
To propagate and maintain plants suitable for use in programs, to reintroduce species into the wild, or to reinforce wild populations.
Since the late eighties, the CPC and the Botanic Gardens Conservation International (BGCI) have instituted standards for documentation, study, and maintenance of rare plant collections at ex-situ sites (BGCI 1993; Wieland 1995).
HORTICULTURAL RESEARCH OF H. DASYCALYX
There is very little information regarding the cultural and maintenance needs of nursery-grown H. dasycalyx for establishment in wildland situations has been published. This information is needed because horticultural treatments, such as fertilization and mulching, may positively affect survivorship, establishment and biomass and reproductive vigor (Poincelot 1980, Vitousek 1982, Merwin and Stiles 1994, Boodley 1996). Plants require an adequate nutrient supply for proper growth, sexual maturity and senescence (Barbour, et al 1987). In the case of some ex-situ habitats, “mitigation” introduction sites, and re-introduction locations which have incurred ground-disturbing activities, soil nutrients may be in short supply or out of balance (Vitousek 1982). Nitrogen is usually the limiting nutrient in many communities, particularly following disturbances. Other cultural treatments such as mulching can maximize growth and availability of essential plant nutrients (Merwin and Stiles 1994). Poincelot (1980) characterizes mulches as the ultimate conservation agent: mulches increase soil moisture retention, mitigate soil erosion, provide a temporary barrier to competition, and mediate soil temperature.
Generally, optimizing plant growth by whatever means, can give the target plant an initial competitive edge over other plants already established in the community. Response of plant vigor in the rare H. dasycalyx during the first year of establishment to fertilization and mulching provides feasibility information for the implementation of ex-situ and re-introduction projects. Equally as important, this study also provides first year anecdotal field observations on inter-specific competition, microhabitat differences regarding moisture regime, and herbivory at this ex-situ location.
LITERATURE CITED
Affolter, J. M. 1997. Essential role of horticulture in rare plant conservation. HortScience, 32(1):29-34.
Ashton, P. S. 1987. Biological considerations in in situ vs ex situ plant conservation. Pp.117-130 in Botanic Gardens and the World Conservation Strategy (D. Bramwell, O. Hamann, V. Heywood, & H. Synge eds.), Academic Press, Inc., Orland, FL, xxxix+367 pp.
Barbour, M.G., J.H. Burk, & W.D. Pitts. 1987. Terrestrial plant ecology. Benjamin/Cummings Publishing Company, Inc., Menlo Park, CA, xiii+634 pp.
BGCI. 1993. Workshop conclusions of the Third International Botanic Gardens Conservation Congress. Botanic Gardens Conservation News, 2(2):41-48.
Blake, S.F. 1958. Two species of Hibiscus from Texas. J. Wash. Acad. Sci., 48:277-278.
Blanchard, O.J. 1976. A revision of species segregated from Hibiscus sect. Trionum (Medicus) de Candolle sensu lato (Malvaceae). Unpublished Ph.D. dissertation, Cornell Univ., Ithica, NY.
Boodley, J.W. 1996. The commercial greenhouse. Delmar Publishers, Albany, NY, xii+612 pp.
Bramwell, D. 1987. Recommendations: Botanic gardens and the world conservation strategy. Pp. 359-361. (O. Hamann, V. Heywood, H. Synge eds.) Academic Press, Orland, FL, xxxix+367pp.
Cahoon, D. R. 1982. Community production and biomass allocation of Hibiscus moscheutos L. (Malvaceae), a brackish marsh dominant. Ph.D. dissertation, University of Maryland, College Park, MD, 120 pp.
Carr, W. R. 1992. Hibiscus dasycalyx 004. Unpublished occurrence report, Texas Natural Heritage Program Files, Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
Center for Plant Conservation. 1991. Appendix. Genetic sampling guidelines for conservation collections of endangered plants. Pp. 225-38, in Genetics and Conservation of rare plants. (D. A. Falk & K. E. Holsinger eds.), Oxford University Press, New York, NY.
Chang, M., L. D. Clendenen, & H. C. Reeves. 1996. Characteristics of a humid climate: Nacogdoches, Texas. Center for Applied Studies in Forestry, College of Forestry, Stephen F. Austin State University, Nacogdoches, TX, xvii+211 pp.
Chang, M. 1997. Pers. Comm. regarding 1996 precipitation data for Nacogdoches, Texas.
Cochis, T. 1964. Studies on problems concerning seed-set in Hibiscus spp. Ph.D. dissertation, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, 88 pp.
Cook, R. E. & P. Dixon. 1989. A review of recovery plans for threatened and endangered plant species: a report for the world wildlife fund. World Wildlife Fund, Washington, DC.
Correll, D. S. & M. C. Johnston. 1979. Manual of the vascular plants of Texas. The University of Texas at Dallas, Richardson, TX, vii+1881 pp.
Cox, P. 1988. Center for Plant Conservation Field Collection Data Sheet for Hibiscus dasycalyx collection of Sept 13, 1988. Texas Natural Heritage Program Files, Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
Creech, D. L. & C. Martindale. 1992. Site analysis of a proposed plant preserve, Nacogdoches County, Texas. Symposium proc. of the Native Plant Society of Texas, October 16-18, Nacogdoches, TX.
Creech, D. L. 1996. SFA arboretum’s three r’s conservation program: rescue, research and reintroduction. Native Plant Society of Texas News. Nov/Dec:6-7.
Dahl, T. E., C. E. Johnson & W. E. Frayer. 1991. Wetlands: status and trends in the conterminous United States, mid-1970s to mid-1980s. U. S. Fish and Wildlife Service, Washington, D. C.
Dolezel, R. 1980. Soil survey of Nacogdoches County, Texas. USDA, Soil Conservation Service, 146 pp.
Doughtry, R.W. & B.M. Parmenter. 1989. Endangered species: disappearing animals and plants in the lone star state. Texas Monthly Press, Austin, TX, x+155 pp.
Eloff, J. N. & L. W. Powrie. 1990. How many plants are needed for ex situ conservation to ensure the subsequent establishment of viable populations? Pp. 397-431, in Proceedings of the International Symposium on Botanical Gardens, Nanjing, 25-28 September 1988. (H. Shen-an, V. Heywood, & P. S. Ashton, eds.), Jaingsu Science & Technology Publishing House, Nanjing, China.
Falk, D. A. 1987. Integrated conservation strategies for endangered plants. Natural Areas Journal, 7:118-123.
Falk, D. A. 1992. From conservation biology to conservation practice: strategies for protecting plant diversity. Pp. 397-431, in Conservation biology: the theory and practice in nature conservation, preservation and management (P. L. Fielder & S. K. Jain, eds.), Chapman and Hall, New York, NY, xxix+507 pp.
Falk, D. A. & P. Olwell. 1992. Scientific and policy considerations in restoration and reintroduction of endangered species. Rhodora, 94(879):287-315.
Fielder P. L. & S. K. Jain. 1992. Conservation biology: the theory and practice in nature conservation, preservation and management. Chapman and Hall, New York, NY, xxix+507 pp.
Giles, F. A., R. M. Keith, & D. C. Saupe. 1980. Herbaceous perennials. Reston Publishing Co., Reston, VA, 356 pp.
Given, D. R. 1987. What the conservationist requires of ex situ collections. P.104., in Botanic gardens and the world conservation strategy (D. Bramwel, O. Hamann, V. Heywood, & H. Synge eds.), Academic Press, Inc., Orlando, FL, xxxix+1-367 pp.
Gordon, R. D. 1994. Translocation of species into conservation areas: a key for natural resource managers. Natural Areas Journal, 14:31-37.
Gould, F. W. 1962. Texas plants—a checklist and ecological summary. The Agricultural and Mechanical College of Texas, Texas Agric. Exp. Sta. Bull., College Station, TX.
Gould, F.W. 1975. Texas Plants–A Checklist and Ecological Summary. Texas Agric. Exp. Sta. Bull. MP-58:1-211, Texas A&M Univ., College Station, Texas.
Guerrant, E. O. 1992. Genetic and demographic considerations in the sampling and reintroduction of rare plants. Pp. 321-344, in Conservation biology: the theory and practice in nature conservation, preservation and management (P. L. Fielder,& S. K. Jain, eds.), Chapman and Hall, New York, NY, xxix+507 pp
Harker, D., S. Evans, M. Evans, & K. Harker. 1993. Landscape restoration handbook. P. B-92. Lewis Publishers, Boca Raton, FL.
Huxley, A., M. Griggiths, & M. Levy. 1992. The new royal horticultural society dictionary of gardening. The Stockton Press, New York, NY.
Klips, R.A. 1995. Genetic affinity of the rare eastern Texas endemic Hibiscus dasycalyx (Malvaceae). Am. J. Bot., 82(11):1463-1472.
Littell, R. C., R. J. Freund, & P. C. Spector. 1991. SAS systems for linear models, Third Edition. SAS Institute Inc., Cary, NC, xviii+329.
Martindale, C. 1990. Site analysis of a proposed plant preserve. Unpublished M.S. thesis, Stephen F. Austin State Univ., Nacogdoches, TX, vii+100.
Maunder, M. 1992. Plant reintroduction: an overview. Biodiversity and Conservation, 1:51-56.
McCartney, R. B. 1995. Marketing wetland natives and endangered plants under federal and state permitting regulations. International Plant Propagators’ Society, 45:617-619.
McMahan, L. R. 1988. Tale of two mallows. The Center for Plant Conservation, 3(4):7.
McMahan, L. R. 1990. Propagation and reintroduction of imperiled plants, and the role of botanical gardens and arboreta. Endangered Species Update 8(1):4-7.
Merwin, I. A., W. C. Stiles, & H. M. van Es. 1994. Orchard groundcover management impacts on soil physical properties. J. Amer. Soc. Hort. Sci., 119:216-222.
Morse, L. E. 1996. Plant rarity and endangerment in North America. Pp. 7-22, in Restoring diversity: strategies for reintroduction of endangered plants (D. L. Falk, C. I. Millar, & M. Olwell, eds.), Island Press, Washington, D. C., xxii+505 pp.
National Wildflower Research Center. 1992. Wildflower Handbook. 2nd Edition. Voyageur Press, Stillwater, MN.
Nemec, K. 1996. Status of H. dasycalyx. Unpublished letter of January 24 to interested parties, USFWS, Division of Ecological Services, Houston.
Nixon, E. S. 1985. Trees, shrubs, & woody vines of East Texas. Bruce Lyndon Cunningham Productions, Nacogdoches, TX, 240 pp.
Nixon, E. S. & J. G. Kell 1993. Ferns and herbaceous flowering plants of East Texas, First Edition, R. Elray Nixon, Las Vegas, NV, ix+453pp.
Orzell, S. L. 1990. Report on areas surveyed for Hibiscus dasycalyx. Texas Natural Heritage Program Files, Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
Poincelot, R. P. 1980. Introduction to applied horticultural science. Prentice-Hall, Inc., Englewood Cliffs, xv+652 pp.
Poole, J. M. 1995. Hibiscus dasycalyx 001. (Trinity County) Updated occurrence record. Texas Natural Heritage Program Files, Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
Raven, P. H. 1987. The scope of the plant conservation problem world-wide. Pp. 19-20, in Botanic gardens and the world conservation strategy (D. Bramwell, O. Hamman, V. Heywood, & H. Synge, eds.), Academic Press, Orlando, FL, xxxix+367 pp.
Reed, P. B. 1986. Wetland plant list of Texas. WELUT-86/W12.43, United States Department of Interior, Fish and Wildlife Service, St. Petersburg, FL.
Robbins, J. T. 1985. The distribution and taxonomy of selected Malvaceae occurring in the southeastern United States and their influence on feeding and ovipositional behavior of the Boll Weevil, Anthonomus grandis Boheman. Ph.D. dissertation, Mississippi State University, Mississippi State, MS, 103 pp.
San Antonio Botanical Center. 1990. Native endangered species propagation: Hibiscus dasycalyx. Unpublished report, Texas Natural Heritage Program Files, Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
Scotts Company. 1996. OsmocoteÒ fertilizer 14-14-14 General Product Information excerpted from H4390 brochure by The Scotts Company Ó 1996, Marysville, OH.
Smith, E. & D. L. Creech. 1995. A propagation and reintroduction strategy for the Neches River rose mallow, Hibiscus dasycalyx. HortSci., 30(4):805.
Snow A. A. & T. P. Spira. 1993. Individual variation in the vigor of self pollen and selfed progeny in Hibiscus moscheutos (Malvaceae). Am. J. Bot., 80(2):160-164.
Snow, A. A. & T. P. Spira. 1994. Evidence for differences among plants in pollen competitive ability: experimental studies of Hibiscus moscheutos (Malvaceae) involving genetic markers. Annual Meeting of the Botanical Society of America, Knoxville, Tennessee, August 7-11. Am. J. Bot., 81(6 suppl.):62-63.
Spira, T. P. 1987. Pollination intensity, seed predation, and reproductive success in Hibiscus moscheutos L. Annual Meeting of the Botanical Society of America, Columbus, Ohio, August 9-13. Am. J. Bot., 74(5):643.
Spira, T. P. 1989. Reproductive biology of Hibiscus moscheutos (Malvaceae). Pp. 247-255 in The evolutionary ecology of plants (J. Bock & Y. Linhart eds.), Westview Press, Boulder, CO.
Spira, T. P., Snow A. A., D. F. Whigham & J. Leak. 1992. Flower visitation, pollen deposition and pollen-tube competition in Hibiscus moscheutos (Malvaceae). Am. J. Bot., 79(4):428-433.
Spira, T. P., A. A. Snow & M. N. Puterbaugh. 1996. The timing and effectiveness of sequential pollination’s in Hibiscus moscheutos. Oecologia., 105(2):230-235
Stebbins, G. L. 1950. Variation and evolution in plants. Columbia University Press, New York, NY.
Texas Natural Heritage Program. 1994. A plan for action to conserve rare resources in Texas. Texas Parks and Wildlife Department, Endangered Resources Branch, Austin, TX.
The Nature Conservancy. 1996. Priorities for Conservation: 1996 annual report card for U. S. plant and animal species. The Nature Conservancy, Arlington, VA.
Vitousek, P. M. 1982. Nutrient cycling and nutrient use efficiency. Amer. Naturalist, 119:553-572.
Warnock, M. J. 1995. Status report on Hibiscus dasycalyx. U. S. Fish and Wildlife Service. Texas Regional Institute for Environmental Studies, Sam Houston State Univ., Huntsville, TX, iv+36 pp.
Weiland, G. D. 1995. Guidelines for the management of orthodox seeds. Center for Plant Conservation, St. Louis, Mo.
Welch, W. C. & G. Grant. 1995. The southern heirloom garden. Taylor Pub. Co., Dallas, TX.
Wilson, E. O. 1988. The current state of biological diversity. Pp. 3-18, in Biodiversity (E.O. Wilson ed.), Nat. Academy Press, Washington, DC, 3:xiii+1-521.
Wise, D. A. & M. Y. Menzel. 1971. Genetic affinities of the North American species of Hibiscus sect. Trionum. Britonia, 23:425-437.

Dr. Creech,
You and your cohorts are to be commended in your study of Hibiscus dasycalyx and all efforts in assisting the renewed growth of this important plant. I have long loved hibiscus. I am surrounded by the flower here in Mexico, although not this particular species. Finally a word of thanks for a post which allows me to recall my science classes at Texas A&M University. I got a C in Biology, a D in Zoology, dropped Chemistry, and struggled through a Horticulture class because of a girl in white shorts who sat on the first row. I think I made a B, but it could have been a D. Anyway, I love hibiscus. I drink it ever day. It helps my heart. One question: what is an “oxbow habitat”? I am well-familiar with “oxbow incidents”, so you might see my confusion. Anyway, well done! Also, I think it was that great scientist Albert Einstein who declared that mankind would be gone from the planet in four years should the hibiscus disappear. We all need to keep that in mind. Thanks. Dr. Duke Miller
LikeLiked by 1 person
Dr. Creech ,
I have both , a Hibiscus dasycalyx and a Hibiscus laevis in my yard . They are planted about 5 feet apart . I got a lot of seeds from Neches River hibiscus . The seeds are reddish and fuzzy . Are these seeds pure Neches or they can be a mix between the two ? I am a Master Gardener in Conroe . Our greenhouse wants to plant my Neches seeds to sell at our Plant sale . I would like to ask you the expert : ” should we or should we not ” because I am afraid we will sell Native Neches River hibiscus , but genetically it is Not . Thank you very much for your help .
Dee Dieu
LikeLike
I wouldn’t worry about it too much. You can tell if they are a hybrid once they start to flower. Hirsute calyx. They could cross with H. laevis depending on how closely they’re to each other. Since they cross naturally “in the wild” I wouldn’t be all that worried about the plant. We have a good number of pure colonies in a number of locations that are far from competing Hibiscus kinfolk . . . I think you could sell it as Hibsicus dasycalyx with som possible crosses. Anything with a wider leaf is suspect.
LikeLike